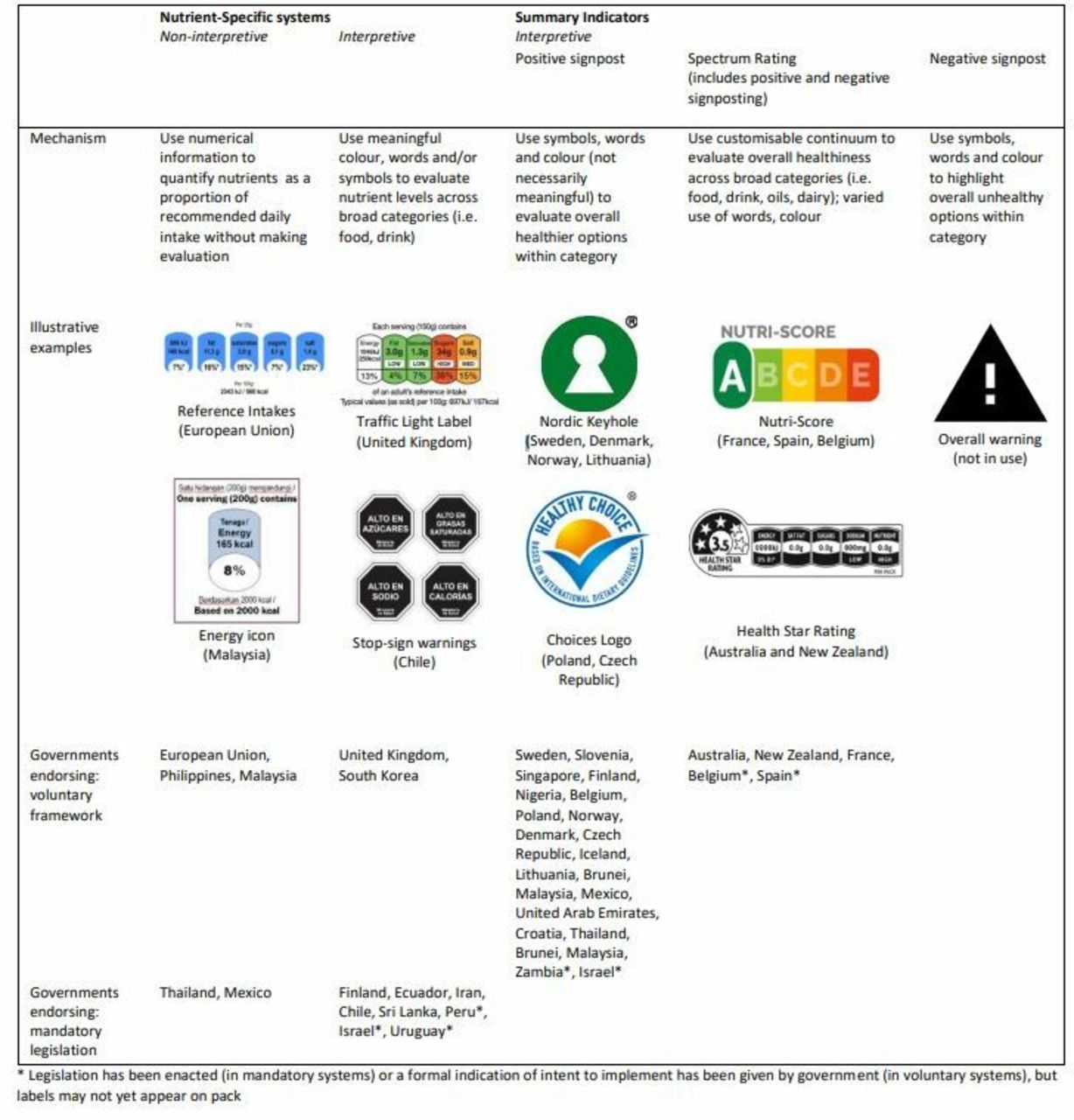
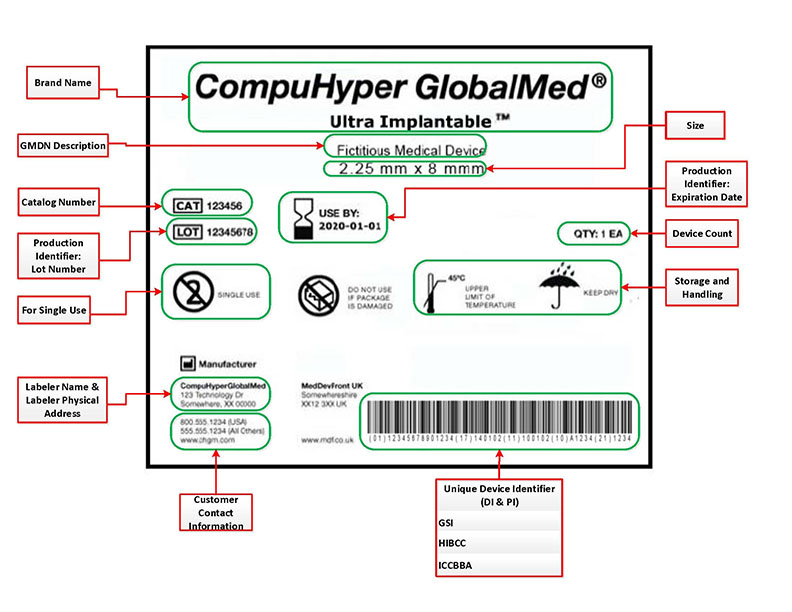
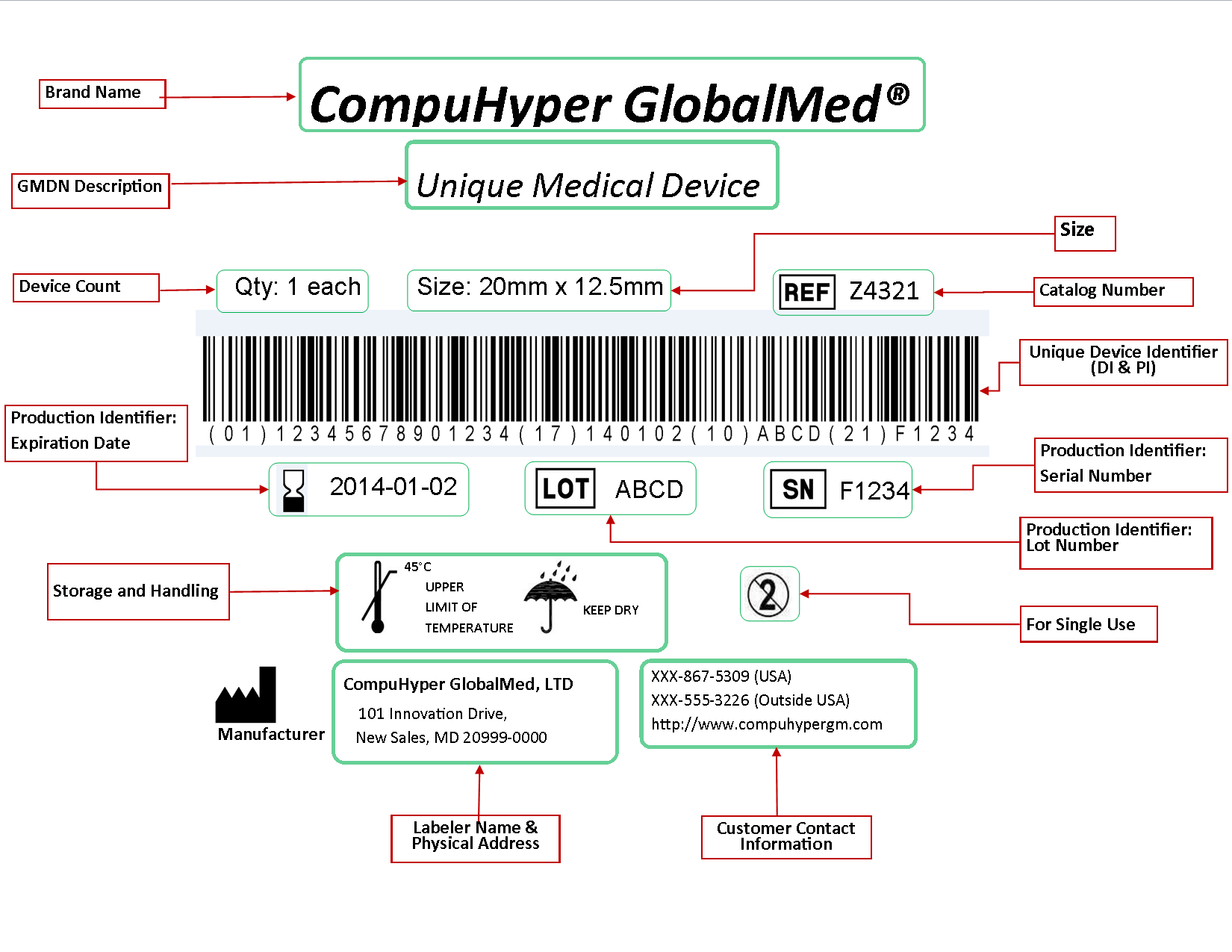
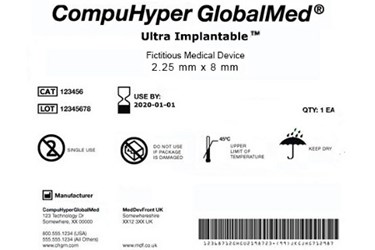
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR): What's the Difference?

Concepts for the Risk-Based Regulation of Clinical Research on Medicines and Medical Devices | Semantic Scholar

ISO 15223-1:2021(en), Medical devices — Symbols to be used with information to be supplied by the manufacturer — Part 1: General requirements